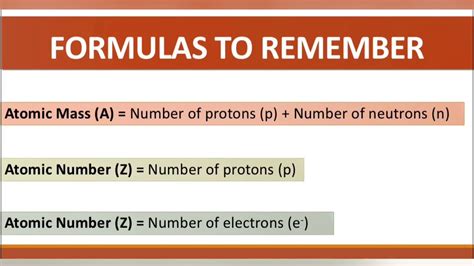
proton neutron electron formula|Protons, neutrons, and electrons in atoms (video) : Cebu You need the atomic number to find the amount of protons and/or electrons, unless you have the amount of neutrons and the .
Join us for a breakdown of the best new sports betting sites in 2024. From A-List to the more obscure, we cover all of the best newcomers. . the platform provides various betting options. Established in 2018, BetMGM has rapidly expanded its reach to over 20 U.S. states, including New Jersey, Arizona, Florida, Nevada, Illinois, and more .
PH0 · Protons, neutrons, and electrons in atoms (video)
PH1 · Number of Protons, Neutrons, and Electrons in an Atom
PH2 · Number of Protons, Neutrons, and Electrons in an Atom
PH3 · How to Find the Number of Protons, Neutrons, and Electrons.
PH4 · How to Find the Number of Protons, Neutrons, and
PH5 · How to Find Number of Protons, Neutrons, and Electrons
PH6 · How To Calculate The Number of Protons, Neutrons, and Electrons
PH7 · How To Calculate The Number of Protons, Neutrons, and
PH8 · Atomic structure
PH9 · 4.4: The Properties of Protons, Neutrons, and Electrons
PH10 · 4.4: Protons, Neutrons, and Electrons
PH11 · 2.6: Protons, Neutrons, and Electrons in Atoms
Inserire il Numero di protocollo (es. 2020/32322) e il Codice Fiscale per ricevere informazioni sullo stato della domanda di Sostegno al Reddito (Sar)
proton neutron electron formula*******We know that the mass number (A) = number of protons + the number of neutrons, and therefore, the number of protons is equal to: p = 35 – 18 = 17, and therefore, the element is Cl. The Number of Protons from Electrons. For a neutral atom, the number of protons and the number of . Tingnan ang higit paIn an easy example, you may be asked to simply determine the number of protons for the given element. For example, how many protons does Ca have? For this, you just need . Tingnan ang higit paThe most important part you will need for calculating the number of neutrons is that together with protons, they make the atomic mass of the given isotope. So, if we have the mass number of the given isotope, we can easily determine the number of . Tingnan ang higit paThe first thing you need to remember here is the charge of the ion is a result of an imbalance between the number of protons and electrons. If it is a cation, then the positive indicates how many more protons it has compared to the number of electrons. . Tingnan ang higit pa Describe the locations, charges, and masses of the three main subatomic particles. Determine the number of protons and electrons in an atom. Write and .
You need the atomic number to find the amount of protons and/or electrons, unless you have the amount of neutrons and the .Protons and neutrons are found in the nucleus, the dense region at the center of an atom. Electrons are found outside the nucleus. Protons are positively charged and have a mass of about 1 u. Neutrons are neutral (have no charge) and also have a mass of .
Protons are found in the nucleus of the atom – the tiny, extremely dense region at the center of the atom. Protons have a positive electrical charge of one (1+) and a mass of . Number of Electrons = Number of Protons. Read More. Unveiling Ions: Counting Protons and Electrons. By Anne Marie Helmenstine, Ph.D. Number of Neutrons = Mass Number - Atomic .In this tutorial, you will learn how to find and calculate the number of protons, neutrons, and electrons in an atom or element. In addition, you will learn about the different .Electrons are extremely small. The mass of an electron is only about 1/2000 the mass of a proton or neutron, so electrons contribute virtually nothing to the total mass of an . This chemistry video tutorial explains how to calculate the number of protons, neutrons, and electrons in an atom or in an ion. It also explains the difference between .
Protons, neutrons, and electrons in atoms (video) In this post, we’ll be going over how to determine the number of protons, neutrons, and electrons in an atom or ion. We’ll first start by discussing what each of .
GCSE; CCEA; Atomic structure - (CCEA) Protons, neutrons and electrons Scientists’ ideas about atoms have changed over time. Today, they agree that atoms have a positively-charged nucleus made of .
The charge of an atom is defined as follows: Atomic charge = number of protons − number of electrons (1.8.1) (1.8.1) Atomic charge = number of protons − number of electrons. As will be discussed in more detail .Neutron. Along with protons, neutrons make up the nucleus, held together by the strong force. The neutron is a baryon and is considered to be composed of two down quarks and one up quark. A free neutron .Table 4.4.1 4.4. 1 gives the properties and locations of electrons, protons, and neutrons. The third column shows the masses of the three subatomic particles in "atomic mass units." An atomic mass unit (amu amu) is defined as one-twelfth of the mass of a carbon-12 atom. Atomic mass units ( amu amu) are useful, because, as you can see, the mass .proton neutron electron formula 🎯 Collège - Lycée Représentation symbolique du noyau de l'atome (A, X, Z) Modèle de l'atome Déterminer le nombre de protons, de neutrons, d'électro.Protons are over 1800 times heavier than electrons. The total number of protons in the atoms of an element is always equal to the atomic number of the element. Neutrons. The mass of a neutron is almost the same as that of a proton, i.e., 1.674×10-24; Neutrons are electrically neutral particles and carry no charge.For example, the atomic number of sodium is 11. Every sodium atom has 11 protons and 11 electrons. It has 11 positive charges and 11 negative charges. The mass number of an atom is its total . 1.10 Calculate the numbers of protons, neutrons and electrons in atoms given the atomic number and mass number. This chemistry video tutorial explains how to calculate the number of protons, neutrons, and electrons in an atom or in an ion. It also explains the differe.Los electrones son las partículas del átomo que se encuentran en la nube que rodea al núcleo. Mientras protones y neutrones se concentran en el núcleo, los electrones se distribuyen en capas en el exterior. Los . The charge of an atom is defined as follows: Atomic charge = number of protons − number of electrons (1.8.1) (1.8.1) Atomic charge = number of protons − number of electrons. As will be .The neutron is a subatomic particle, symbol n or n 0, which has a neutral (not positive or negative) charge, and a mass slightly greater than that of a proton.Protons and neutrons constitute the nuclei of atoms.Since protons and neutrons behave similarly within the nucleus, they are both referred to as nucleons.Nucleons have a mass of approximately .Together, the number of protons and the number of neutrons determine an element’s mass number: mass number = protons + neutrons. If you want to calculate how many neutrons an atom has, you can simply subtract the number of protons, or atomic number, from the mass number. A property closely related to an atom’s mass number is its .

Watch this video to learn how protons, neutrons, and electrons are arranged in atoms, and how the number and distribution of these subatomic particles determine the identity and properties of .
proton neutron electron formula Protons, neutrons, and electrons in atoms (video) # of protons = 17 # of neutrons = 37 – 17 = 20 # of electrons = 17 – 0 = 17 # of protons = 16 (the atomic number is not given, but can be found on the periodic table) # of neutrons = 32 – 16 = 16 # of electrons = 16 – (-2) = 18. Additional Practice. Try these on your own and check the answer below 78 Se 2-39 K + ANSWERS. 34 protons, 44 .
The proton is a more massive (but still tiny) subatomic particle with a positive charge, represented as p +. The neutron is a subatomic particle with about the same mass as a proton, but no charge. It is represented as either n or n 0. We now know that all atoms of all elements are composed of electrons, protons, and (with one .Atoms are the basic particles of the chemical elements.An atom consists of a nucleus of protons and generally neutrons, surrounded by an electromagnetically bound swarm of electrons.The chemical elements are distinguished from each other by the number of protons that are in their atoms. For example, any atom that contains 11 protons is .
Pinay scandal. Newest. Newest Best Most viewed Longest Random. Sa loob iputok sabi ni Manager. Dukit ang libangan ni Sheina. Araw-Araw Vidjakol Pampaalis kay Babe. Nanggigil Si Raprap Sa Aking Malaking Hinaharap. Cavite girl nag uumapaw sa Libog. Papaligayin ka ni Tiffany. Finger video para sa crush niya.Just imagine how existing it is to take part in a true Filipino sex scandal when you’re getting screwed with your attractive partner while she obediently does whatever you say. We bet you've dreamt about it many times. And now, with the help of the Kantotin website, you can become a hidden spectator of somebody's intercourse in a few mouse .
proton neutron electron formula|Protons, neutrons, and electrons in atoms (video)